SOLVED: You set up a reaction with Gallium metal in excess hydrochloric acid at 28 degrees Celsius It reacts in the following: 2Ga(s)+6HCIaq)-2GaClylaq)+3H2lg) You collect 160 mLs and 0.966 atm of hydrogen

The given diagrams show the reactions of three metals with dilute hydrochloric acid.What are metals P, Q and R ?P Q R

Unlocking the Potential of Hydrochloric Acid in Metal Processing and pH Regulation: It's a HCl of a Job – Alliance Chemical

HCL Series Hydrochloric Acid for Metal Processing - China 7647-01-0, Muriatic Acid | Made-in-China.com

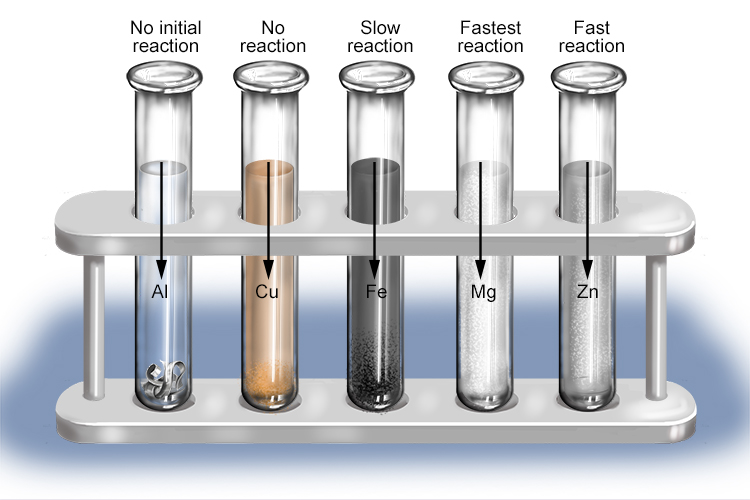
Metals Reacting with Water & Acids (2.4.1) | Edexcel IGCSE Chemistry Revision Notes 2019 | Save My Exams

Question Video: Identifying Which Metals Form a Salt When Reacted with Hydrochloric Acid Solution | Nagwa